New Investigator Toolkit
Are You New to the IRB Application Process?
Let us walk you through each step and assist you with your first University of Utah human subjects research application.
Begin by selecting the type of study you plan to submit.
Next, learn more about how to prepare to submit your IRB application.
Finally, let us walk you through how to submit an IRB application in ERICA system.
What Type of Study are you Submitting?
Learn about the review process for your study. We will walk you through how to submit a single-site or multi-site study.
Single Site
If you plan to conduct a study with:
- One participating site, and
- You will use the University of Utah IRB for approval of the study
The process for preparing your study proposal, submitting in ERICA, and obtaining IRB approval is below:

- Go to the next section on this page to learn more about the Pre-Application Process. It is important that you properly prepare your study documents before you begin
your IRB application to ensure your application is as complete and thorough as possible.
- Go further down the page to learn about Submitting a New Study Application in ERICA.
- Learn more about the other committees at the University of Utah that may need to review
your application on the Ancillary Requirements page.
- Pre-Review is the first stage of the IRB review process. Your application is assigned to an IRB staff member
for a preliminary review. The staff member will carefully review your application
for common errors and request clarifications/revisions as needed.
- Board Review is the stage where IRB board members are reviewing and considering whether to approve
your application.
- During Final Processing, the IRB staff are processing your study file and preparing your final determination
letter.
- After you recieve intial IRB approval, there may be Ongoing Review requirements for IRB submissions or reports during the course of your study. Please read your letter carefully and familiarize yourself with these requirements.
Multi-Site, University of Utah as the SIRB
If you plan to conduct a study with:
- Multiple participating sites, and
- You will use the University of Utah IRB for approval of the study
The process for preparing your study proposal, submitting in ERICA, and obtaining IRB approval is below (click on the image to view it larger):
When you are ready to submit your application, the IRB review process works as follows:

- Go to the next section on this page to learn more about the Pre-Application Process. It is important that you properly prepare your study documents before you begin
your IRB application to ensure your application is as complete and thorough as possible.
- Go further down the page to learn about Submitting a New Study Application in ERICA.
- Learn more about the other committees at the University of Utah that may need to review
your application on the Ancillary Requirements page.
- Pre-Review is the first stage of the IRB review process. Your application is assigned to an IRB staff member
for a preliminary review. The staff member will carefully review your application
for common errors and request clarifications/revisions as needed.
- Board Review is the stage where IRB board members are reviewing and considering whether to approve
your application.
- During Final Processing, the IRB staff are processing your study file and preparing your final determination
letter.
- After you recieve intial IRB approval, there may be Ongoing Review requirements for IRB submissions or reports during the course of your study. Please read your letter carefully and familiarize yourself with these requirements.
Multi-Site, External SIRB
If you plan to conduct a study with:
- Multiple participating sites, and
- You will use an IRB other than the University of Utah IRB for approval of the study
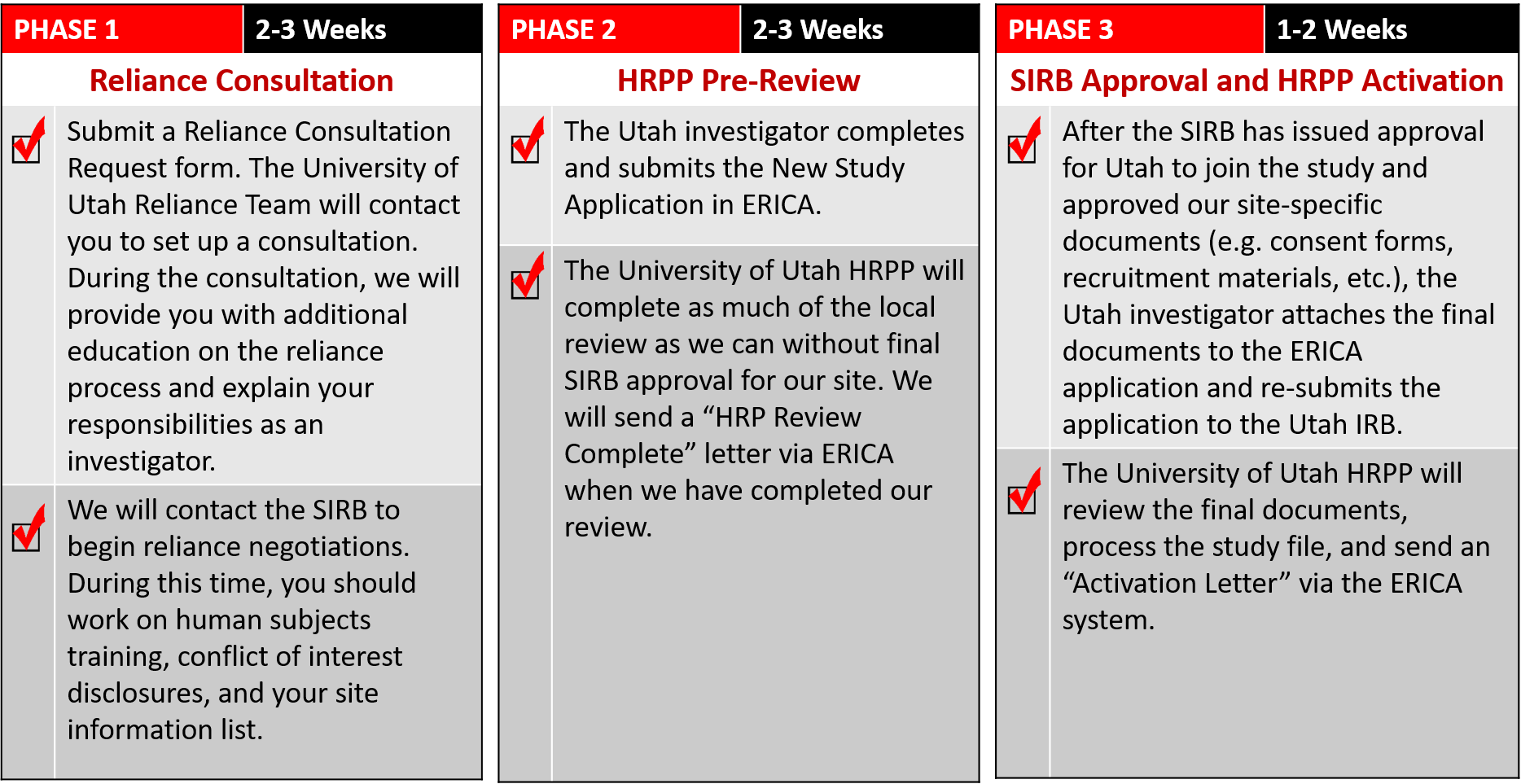
The process for preparing your study proposal, submitting in ERICA, and obtaining IRB approval is below (click on the image to view it larger):
When you are ready to submit your ERICA application, the IRB review process works as follows:

- Go to the next section on this page to learn more about the Pre-Application Process. It is important that you properly prepare your study documents before you begin
your IRB application to ensure your application is as complete and thorough as possible.
- Go further down the page to learn about Submitting a New Study Application in ERICA.
- Learn more about the other committees at the University of Utah that may need to review
your application on the Ancillary Requirements page.
- The University of Utah HRPP Review process involves (Human Research Protection Program) will completing as much of the
local review as we can without final SIRB approval for our site.
- We will send a "HRP Review Complete" letter via ERICA when we have completed our review.
- During Final Processing, the IRB staff are reviewing the final documents and processing your study file.
We will send an Activation Letter via the ERICA system when this final check is complete.
- After you recieve intial IRB approval, there may be Ongoing Review requirements for IRB submissions or reports during the course of your study. Please read your letter carefully and familiarize yourself with these requirements.
Pre-Application Process
Follow these steps to prepare to submit an IRB application. Click on each button below to learn more.
Step 1: Familiarize Yourself with the Resources Available to You.
| This page has been developed as a central index for the IRB website. It includes links to several guidance topics that may be applicable to your study. | |
| Study team toolkit | This page includes link to several IRB resources that may be useful for study teams. |
| If you are not associated with the University of Utah, or if you will be engaging in research at a location that is not part of the University of Utah and would like our IRB to serve as the IRB of record for your research, our External Institutions policy may be applicable. | |
| consent guidance | The IRB has an extensive library of guidance to help you design and document your consent process. |
| Clinical Research SOPs | Standard Operating Procedures (SOPs) for clinical research at the University of Utah. |
STEP 2: Write a Summary of your Study.
Write out and organize your research plan. You will need to have a clear design outlined before you will be ready to submit your application to the IRB. The following information is essential for completion of the application. ALL INVESTIGATORS should be prepared to provide this information:
- Background and Introduction. Identify the research area being studied and provide a review of the literature that
provides the basis for understanding the objectives of the study. This review should
be written such that scientists outside the investigator's area of expertise can understand
the issues involved. Any information about previous research related to this study
involving animals and/or humans should be summarized. Include studies on pregnant
animals if the research is conducted on pregnant women, fetuses, or neonates.
- Objectives. The objectives should be stated in such a way that the reader can determine the appropriateness
of the study design below. If appropriate, state the specific hypotheses being tested
and/or study aims.
- Participant Selection Criteria. Participant-entry criteria should be as detailed as necessary to define the patient
population under study and, for clinical studies, to reduce confounding treatments
or diseases. Precise criteria for age, gender, or any other factors (e.g., in a clinical
study: diagnoses, extremes in signs or symptoms, etc.) should be included. Specific
exclusion criteria should be listed which could interfere with the study or which
place participants at risk during the study.
- Design. A simple statement of the design methodology proposed to test your hypothesis(es)
should be included. Randomization and control methods should be stated. Of primary
importance is clearly showing how the trial design will collect the study data and
lead to the analysis and interpretation proposed. Any interim analysis or criteria
for stopping a clinical trial should be stated. For studies not proposing experimental
design include detail about the scientific methods to be employed.
- Study Procedures.This section of the protocol should state both the chronological flow of the study
and the procedures/activities that the participants must undergo. The investigational
activities, treatments, or procedures must be clearly detailed as to how and when
they will be performed. For clinical studies, a distinction should be made between
the procedures for treatment evaluation versus procedures for safety evaluation. Treatment
endpoints must be defined as well as interim procedures for dealing with adverse events.
Schematic diagrams may be helpful for understanding the flow of a study.
- Standard of Care vs. Research-Related Procedures. If applicable, please separate and explain what proposed procedures for this study are considered standard of care and which ones are strictly research-related.
- Data and Safety Monitoring Plans. The information provided to the IRB should describe the process and mechanisms in
place for assuring 1) the oversight of data integrity, and/or 2) the safety of research
participants.
- Statistical Methods, Data Analysis, and Interpretation. The anticipated methods to be used for analysis and interpretation of the data should
be stated. Naturally, these methods must compliment the design of the trial and the
nature of the data which are being collected. The factors in the trial that determine
the proposed sample size (e.g. power) should be stated.
- Administrative Responsibilities. Specify the resources available to conduct the research including qualified personnel,
equipment, space, and what facilities will be involved. Include an explanation of
the methods for maintaining confidentiality of the study data.
- Recruitment. If applicable, describe methods of participant recruitment which will be used to recruit
participants such as newspaper/internet advertisements or flyers. Attach all recruitment
materials to the application for review.
- References and Appendices. Citations from the literature should be included in the Background/Introduction section above and the references listed here. Other supporting information, such as your own publications, should be submitted if you feel it would allow a deeper understanding of the project.
The following information should also be prepared, as it applies to your study:
- Control of Investigational Devices/Drugs. If this project involves an investigational drug or device please provide a plan as
to how you will control, store, and dispense investigational drugs/devices to ensure
they are only used by the qualified investigator(s) for this study and the participants
enrolled in this research project.
- Communication Plans for “Multi-Center” Studies (i.e. multiple sites around the city,
state, nation, or world with a PI other than the local PI designated at each site).If you are the lead investigator for this study, or the University of Utah is the
lead site for this study, please describe the management and communication among sites
of information obtained in this research that may be relevant to the protection of
research participants, such as: unanticipated problems involving risks to participants
or others, interim results, and protocol modifications.
- Please attach an IRB approval from each participating site to the Documents and Attachments
page of the application under “Other Documents”.
- If the site does not have its own IRB of record, please contact an IRB Administrator for assistance.
- Please include contact information (address, phone, and email) for each participating
site. This can be attached separately to the Documents and Attachments page of the
application under “Other Documents”.
- Please attach an IRB approval from each participating site to the Documents and Attachments
page of the application under “Other Documents”.
- Participating Sites Outside the University of Utah (i.e. multiple sites around the
city, state, nation, or world where the local PI runs the study at each site). This section should discuss which other institutions are participating in the study
for which you, as the PI, are responsible. Please describe the procedures, provisions
and resources in place at the participating institutions to protect the safety of
participants, and how unanticipated problems will be communicated to the PI and the
University of Utah IRB. If the participating site is not adequately equipped to handle
safety concerns, please explain the procedures and plan in place for the PI to respond
to any such occurrences.
- Please include contact information (address, phone, and email) for each participating site. This can be attached separately to the Documents and Attachments page of the application under “Other Documents”.
- Please attach an IRB approval (if the site has its own IRB) or signed letter of support from each participating site to the Documents and Attachments page of the application under Other Documents.
STEP 3: Determine What Level of Consent Your Study will Require, Then Design and Test Your Consent Process.
There are many different ways to obtain consent for research; you will need to determine which method is appropriate for your study. You are encouraged to visit our Consent Guidance page as you design your process.
STEP 4: Register in ERICA.
The Electronic Research Integrity and Compliance Administration (ERICA) system is the primary electronic resource for the IRB review process. ERICA is used to enter, submit, track review, and approve all studies submitted to the IRB. For information on completing this process, please see the ERICA Access Instructions page.
Step 5: Complete Human Subjects Research Training.
University of Utah investigators and study staff who conduct human subject research must complete an IRB approved method of human subjects research training and a Good Clinical Practices (GCP) course before the IRB will approve their project. There are several options available to investigators to satisfy this requirement. Please review the Human Subjects Research Training page for more information.
Step 6: Determine Which HRPP Committees will Also Need to Review your Application.
In order to receive IRB approval, it may be necessary to provide information to and/or receive ancillary approval from one of the other committees within the University of Utah Human Research Protection Program (HRPP).
Learn more about these groups here: Ancillary Requirements
Each applicable committee has their own requirements for submission. Please check with the applicable committee for their current requirements.
The University of Utah IRB follows and adheres to the University’s Conflict of Interest policy (U Pol 1-006). The principal investigator and all faculty, staff, postdoctoral appointees, residents or students, whether paid by the University or not, who are responsible for the design, conduct or reporting of research conducted in whole or in part at the University must complete a Conflict of Interest Disclosure Form within the ERICA online system.
Submitting a New Study Application in ERICA
Investigators applying for any initial approval of a proposed research protocol must submit a completed ERICA New Study application.
The ERICA system uses a “smart form” application which requires specific information based upon the responses of the applicant. For example, the New Study application includes a specific page for placebo-controlled trials. This page will only generate for the investigator if they indicate earlier in the application that the study involves placebo. Applications which are missing responses in required fields cannot be submitted electronically.
Additional forms within the ERICA system and documents created outside of the ERICA system may be required to complete an ERICA New Study application. Forms should be completed within ERICA as indicated. Documents should be attached to the “Documents and Attachments” page of the ERICA New Study application. See the Investigator Guidance Series: Required Documents and Forms for IRB Applications for a list of documents and forms necessary for applications.
Instructions:
- Log onto ERICA
- Click on the IRB Studies tab
- Click on the Create a New Studies Application button
- Complete the new study application entirely
- Attach required and supporting documents to the Documents and Attachments page
- Submit the new study application to the IRB for review
Note: It is the PI's responsibility to oversee and ensure proper completion of information to the IRB. This includes the submission of all applications pertaining to the study, as well as revisions requested during the IRB review of an application.
Do You Have Suggestions for Improving this Page?
Let us know!
If you have suggestions for additions or enhancements for this page or any page on the IRB website, please tell us: email us