Final Project Reports
The completion or termination of a study is a change in activity and must be reported to the IRB. At the time a study is complete or discontinued, the investigator must submit a Final Project Report with the following information included:
- Total number of subjects
- Amendments since last approval
- Problems/complications and subject withdrawal, as applicable
- Study results, if available
- Sponsor/accounting information
Final Project Report Criteria
- The study is permanently closed to enrollment of new participants;
- You are no longer continuing to or planning to perform interventions (e.g., tests, physical or psychological exams, administer medications or treatments, etc.)on participants in order to gather data about them (including collection of data for follow-up);
- You are no longer continuing to or planning to interact (e.g., letters, phone calls, interviews, re-contacting, etc.) with any participants in order to gather data about them;
- You are no longer continuing to or planning to gather any private identifiable information about participants.
- Data analysis is complete OR if you are continuing or planning to analyze data, the data does not contain participant identifiers or a link/code to identify study participants;
- If applicable, your sponsor/monitor has conducted the official close-out visit and will no longer require access to participant records or contact with the participants;
- If applicable, documentation from the sponsor that all correspondence and queries
related to the study have been addressed and the investigator's participation in the
study is complete.
If these criteria are not met, the IRB application should NOT be closed using a Final Project Report; the investigator must apply for continuing review to allow for continued research activities. If the study is closed before these criteria are met, a new study application must be submitted before continuing with research activities. The investigator may not conduct research activities until he/she has received IRB approval to do so.
NOTE: Please note that if the study does not not expire (e.g. the study is Exempt, or the IRB has determined that CR is not required), a CR is not required to renew the IRB approval. A FPR should still be submitted when the study meets the FPR criteria noted above.
Do you have a Faculty Sponsor? If your study is has a Faculty Sponsor, please make sure your Faculty Sponsor also submits your FPR to the IRB. ERICA will send automatic reminders to you until the FPR application is submitted.
Submitting a Final Project Report in ERICA
- Open the study in ERICA.
- Click on the Continuing Reviews tab.
- Click on the Create button.
- Answer the questions on the first page. Keep in mind that your answers must align
with the criteria mentioned above in order for the application to take you to the
Final Project Report.
learn more about starting the final project report in erica - Answer "Yes" on the Final Project Report page.
- Complete the remaining pages of the final project report entirely.
- Submit the final project report to the IRB for review.
Note: It is the PI's responsibility to handle the submission of information. This includes
the submission of all applications pertaining to the study, as well as revisions requested
during the IRB review of an application.
Administrative Study Closure
If the Principal Investigator has not completed study enrollment and/or procedures and the study expires, they must immediately cease enrollment and all other activities related to the study. If a continuing review application has not been submitted before the study expires, the study will be administratively closed. In order to continue with the study, a new study application must be submitted to the IRB. The investigator may not continue until they have received IRB approval to do so.
Final Project Report
The Final Project Report (FPR) is part of the Continuing Review (CR) Application. On the first page of the CR Application, you will be asked questions regarding the status of the study. Based on your answer, ERICA will determine whether the study is eligible to submit a FPR.
If the study is eligible to submit a FPR, when you click "Continue" on the first page,
the application will automatically move to the first page of the Final Project Report
Application.
ERICA Application Steps
|
STEP 1: Enrollment status of the study. If you indicate that enrollment is on hold or closed, more questions will reveal asking you to provide more details. |
 |
|
STEP 2: Remaining research activities. If your study is eligible to submit a FPR, the last question will ask you if you would like to submit a FPR and close the study. |
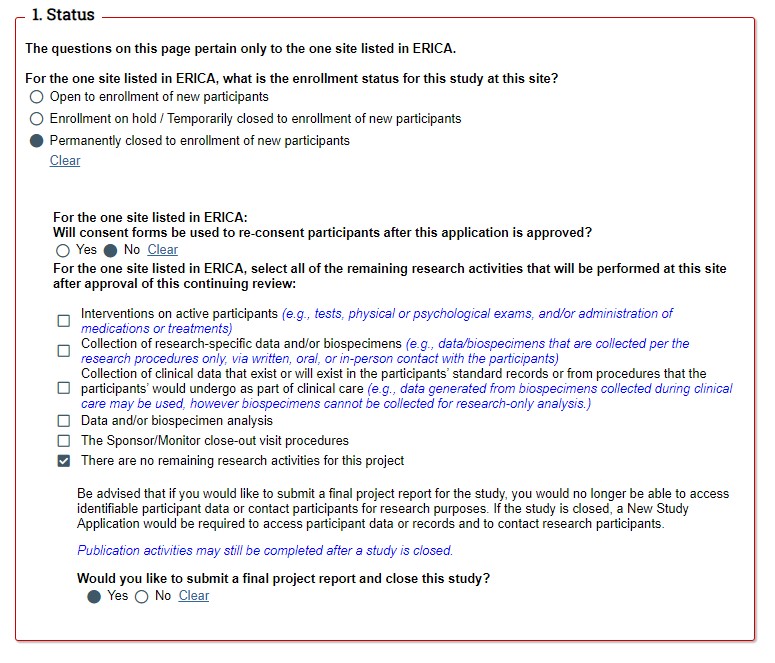 |
|
STEP 3: Complete the Final Project Report (FPR). If you decide to close the study, the first page of the FPR will automatically generate when you click "Continue". Complete the remainder of the FPR according to the on-screen instructions in ERICA. Don't forget to have the PI submit the FPR to the IRB. |
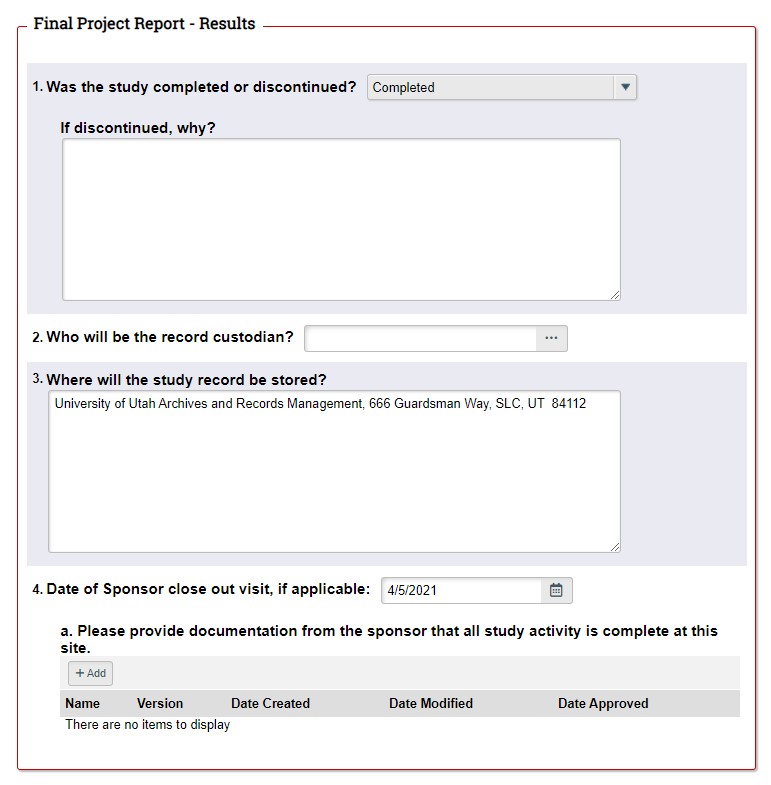 |
