News & Announcements
Listserv
Are you a member of our listserv? The IRB sends out all announcements and updates on the IRB listserv. If you would like to be added to this email list:
- Send an email to sympa@lists.utah.edu.
- In the "Subject" line, type "Subscribe irbalert <<yourname> >".
- <<yourname> > should be your first and last name (no space).
-

Notification for ERICA Users on MacOS
The IRB has been notified that that there is an urgent usability issue related to ERICA for individuals using Mac computers.
-

Update on Federal Funding: Supporting Our Research Community
We recognize the concerns stemming from the recent Office of Management and Budget (OMB) memorandum (M-25-13), which calls for a temporary pause on federal financial assistance programs, including grant disbursements and new awards, effective January 28th, 2025 at 5:00pm EST.
-

Upgrades to Business Relationship Reporting System
The platform for the University of Utah's Business Relationship Reporting ("BRR") financial disclosure system was upgraded on January 22, 2025, to a revised system supported by the Office of the Vice President for Research (VPR).
-

New IRB Staff Member
Please join us in welcoming a new member to the IRB staff team.
-

The Research Post
Visit the U Research Post to subscribe for campus-wide research announcements, news, and updates.
-

SIRB Review Fees
The University of Utah IRB will be enforcing our Single IRB Review fees policy in a new way beginning April 1, 2024. We ask that you help to share this with researchers in your department/division as we prepare to make this change in practice. For studies that cannot pay the expected Single IRB Review fees throughout the course of the study, the IRB will bill the study’s department for these fees.
-

New IRB Staff Members
Please join us in welcoming two new members of the IRB staff team.
-
VPR Statement on the Use of Artificial Intelligence (AI) in Research
Artificial intelligence (AI) is technology that is changing the landscape of higher education in terms of teaching and research. For research activities, policies and guidelines about the use of AI, specifically in terms of writing and reviewing manuscripts, papers, and grant proposals, are evolving across federal agencies, journals, and academic institutions. It is up to investigators, project staff, and students to be aware of the applicable policies and guidelines surrounding the use of AI programs and tools, and question how reliable they are for use in the research environment.
-

Utah's New Data Privacy Laws
Utah is at the frontier of consumer privacy, becoming the first U.S. state to pass a law focused on social media privacy rights for children and the fourth state to enact comprehensive consumer data protection legislation.
-

Updated IRB Fee Schedules, Effective July 1, 2023
IRB fees will be changing effective at the beginning of the fiscal year. The changes will affect industry-sponsored new studies as well as SIRB fees for multi-site studies.
-
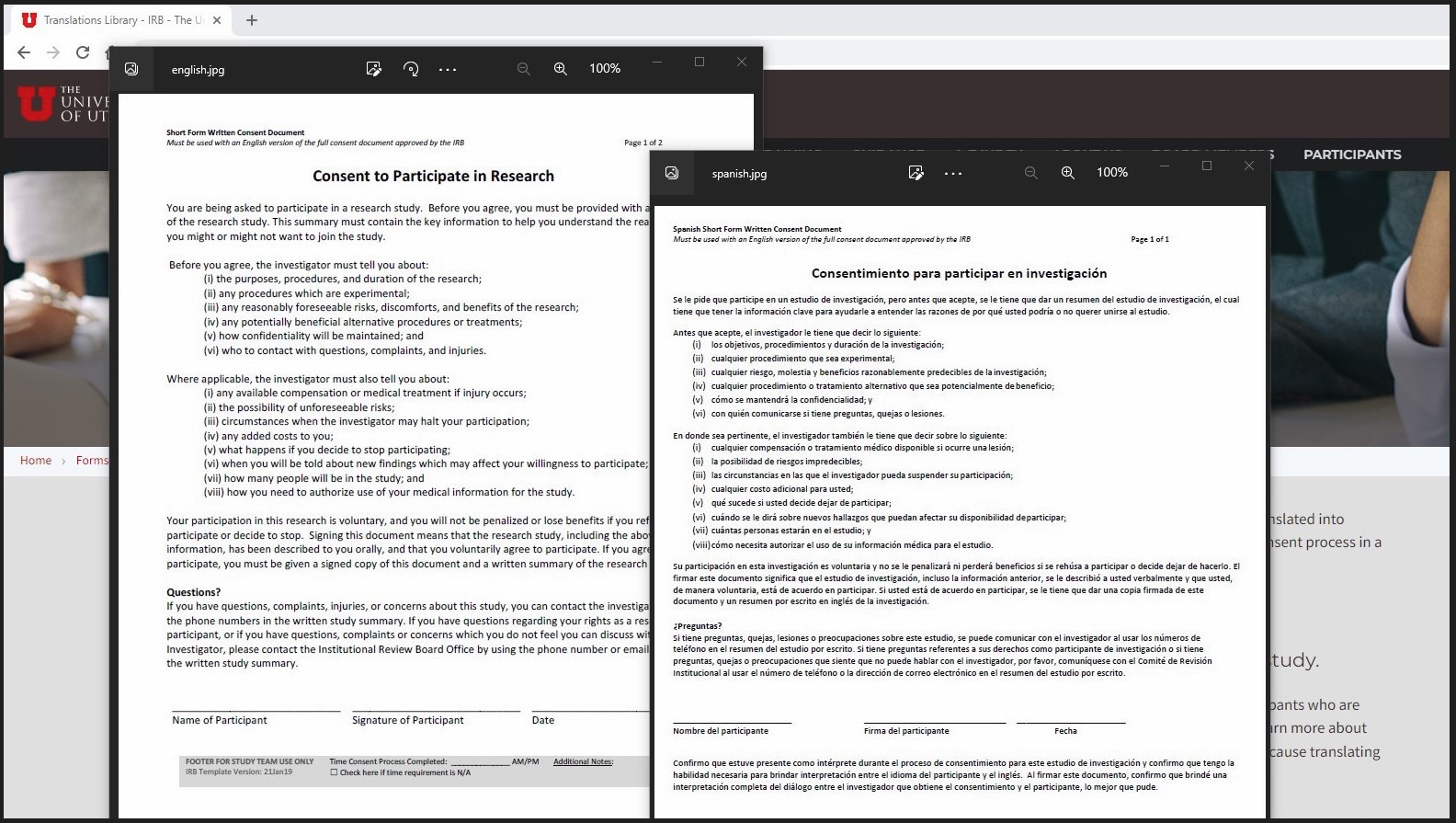
New Policy: Including People Who Speak Spanish
All new, prospective studies with participant interaction conducted by University of Utah investigators within the state of Utah submitted after July 1, 2023, must adhere to a new policy to receive IRB approval: "Inclusion of People Who Speak Spanish in Research".
-

New IRB Staff Members
Please join us in welcoming a new Administrator and two new Coordinators to the IRB staff team.
-

HRPP Progress Updates
HRPP Progress Updates are due before the annual anniversary date of the study's initial approval or exemption determination from the ERICA system. If the PI does not submit the HRPP Progress Update by the annual anniversary date of the study's initial approval/exemption, the study will be administratively closed.
-

New IRB Administrator
We are excited to announce a new addition to the IRB Administrator team.
-

New IRB Operations Manager
We are pleased to announce that April Edwards has accepted the position as the new IRB Operations Manager.
-

New IRB Clerk
Please join us in welcoming a new IRB Clerk to our team.
-

New IRB Associate Director
We are pleased to announce that Annie Risenmay has accepted the position as the new IRB Associate Director.
-

New IRB Coordinator
Please join us in welcoming a new coordinator to the IRB staff team!
-

New IRB Administrator
Please join us in welcoming a new administrator to the IRB staff team!
-

New IRB Coordinator
Please join us in welcoming a new coordinator to the IRB staff team!
-

ERICA Planned Downtime, April 21-24, 2022
ERICA will be offline from Thursday, April 21 at 5:00 PM through Sunday, April 24th, 2022. Please plan your IRB submissions accordingly, and note that the ERICA system will also be unavailable to IRB staff and board members during this time.
-

New IRB Coordinators
Please join us in welcoming two new coordinators to the IRB staff team.
-

New IRB Clerk
Please join us in welcoming Katherine Frederickson to the IRB staff team.
-

New IRB Administrators
Please join us in congratulating Hannah Baley and Anthon Wood on their promotions to IRB Administrator.
-

New IRB Administrator
Please join us in welcoming Heather Brown to the IRB staff team! Heather will be joining our Quality & Compliance Team.
-

Annual HRPP Progress Updates
The IRB and Human Research Protection Program (HRPP) have initiated a new requirement for Annual HRPP Progress Updates in the ERICA system.
-

New IRB Chairs Announced
We are excited to announce that beginning July 1st, Dr. Mark Eliason, Dr. Dick Lemons, and Dr. Ruth Gerritsen-McKane will begin their service as Chairs of the IRB. Together with our Vice Chairs and IRB management staff, the IRB Executive Committee will be exploring several exciting initiatives as we move into FY 2022.
-

New IRB Clerk
Please join us in welcoming Karla Padilla Amador to the IRB staff team! Karla will be joining our Administrative Support Team and will be helping with our front desk phones, and managing CITI trainings and fee invoices.
-

New IRB Coordinator
Please join us in congratulating Militza Martinez on her promotion to IRB Coordinator. Militza will be taking over Panel 2, and she will be pre-reviewing Continuing Reviews and Amendments.
-

Open Position: IRB Chair
The University of Utah Institutional Review Board (UU IRB) is seeking individuals to fill two open IRB Chair positions.
-

Winter Break - IRB Closure
In alignment with the University’s announcement and intent of the Winter Break Administrative Leave to allow time off to rest and relax, University of Utah IRB will be fully closed from December 24, 2020 through January 3, 2021, reopening on Monday, January 4, 2021.
-

ORIC Annual Report
The Office of Research Integrity and Compliance is proud to release the 2019-2020 ORIC Annual Report. In here, you’ll find information on new ORIC updates, department highlights, new resources and training, and a letter from Dr. Erin Rothwell, Associate Vice President for Research.
-
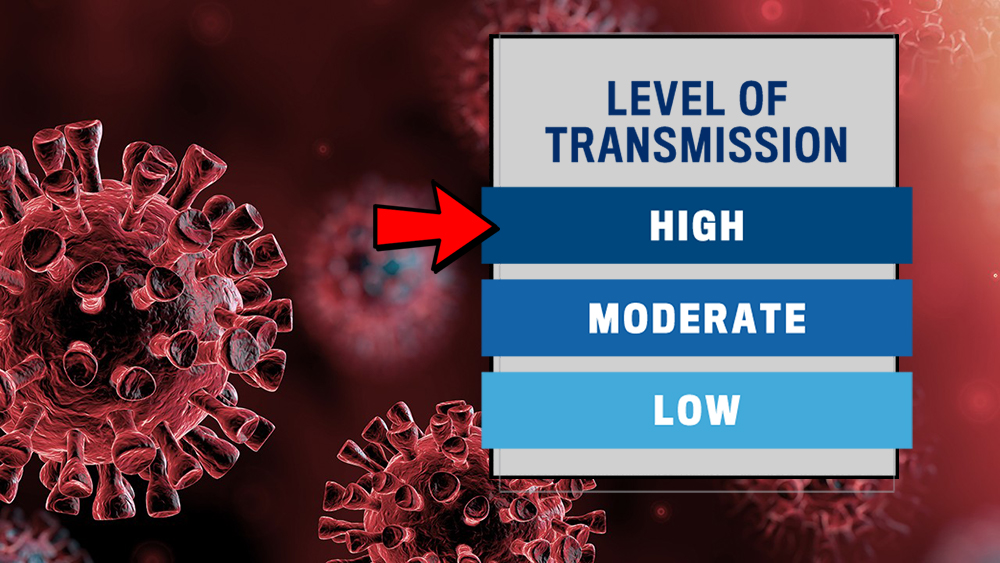
Operations Update
Our level of transmission is currently at “HIGH”. The new transmission index has not changed allowable research activities on campus. We are still currently under “Limited Research Activities Status”.
-
IRB & Research Preparations for COVID-19
The IRB is heeding current guidelines from University leaders in response to the COVID-19 outbreak. This announcement is to provide the research community with information regarding the review and conduct of human subject research during this time.
-

ERICA System Update Complete
The ERICA online system was updated over the weekend. A video is available to walk you through the changes.
-

Investigator Guidance Update
We have posted several updates to our Investigator Guidance Series.
-

New University Rule Regarding Use of Biospecimens
Effective August 26, 2019, the University of Utah has issued a policy regarding the use of biospecimens. Rule 7-002A defines what constitutes a biospecimen and what qualifies as University research.
-

Website Update
The IRB website has been converted to the latest University of Utah template. New features include a floating menu bar (click “Menu” in the top corner to access) and enhanced search capabilities. The ERICA button is in the floating menu, which is accessible from any page on the site.
-
New Video Available: Budgeting for SIRB Review Fees
The IRB's new instructional video guides you through some of the nuances of single IRB review fees and when you may need to think about these issues prior to initiating a grant or review with the IRB.
-

New Translation Library
The IRB is excited to announce that our new short form translation library is now available.
-

New Administrators and Coordinators
Please join us in congratulating Emma Tkachuck and April Edwards in their recent promotions to the Administrator team. We would also like to welcome our two new Coordinators, Muriel McGregor and Anthon Wood.
-

New Research Education Website
The Research Education Department's new website is now active at http://education.research.utah.edu. Several free continuing education and training opportunities are available.
-

Changes to Investigator Guidance
The University of Utah IRB continually strives to provide information and training to the research community. Additionally, we regularly review our guidance documents to make updates as necessary.
-

Symposium: The Future of Clinical Research at the University of Utah, CHALLENGES AND OPPORTUNITIES
Please join fellow researchers, leaders and administrators as we prepare for future challenges and opportunities in clinical research.
-

Updated IRB Fee Schedule
The IRB Review Fees for an Individual Site, which are typically only charged to industry-sponsored studies, have increased. Additionally, fee information for Single IRB (sIRB) Review Services for Multi-Site Studies is now available on the IRB website.
-

January 21st is the Go-Live Date for the New Version of the Common Rule
The Common Rule regulations have been revised, with a general compliance date of January 21st, 2019.
-

IRB Coordinator
Please welcome Rachel Snow as our newest IRB Coordinator.
-

New Consent Guidance and Revised Common Rule
In anticipation of the revisions to the Common Rule that go into effect on January 21, 2019, the IRB is excited to announce changes to the informed consent guidance we offer the research community.
-

IRB Administrator
Please welcome Lindsey Byrd as our newest IRB Administrator.
-

IRB Coordinator
Please welcome Emma Tkachuck as our newest IRB Coordinator.
-

Short Form Consent Documents and Interpreter Signatures
In order to clarify the exact role that the interpreter plays as a witness to the Short Form consent process, the University of Utah IRB has updated the Short Forms to include a statement with the interpreter signature lines.
-

Access to Training Reports in ERICA
ERICA users can now access training reports in ERICA for any individual with an ERICA account.
-

Changes to Screening & Recruiting Policies
Effective June 2018, the University of Utah IRB (UUIRB) updated its screening and recruiting policies and procedures.
-

Transition from Campus Orders to UShop for IRB Fees
Beginning June 29, 2018, the IRB will no longer accept payment for fees using a Campus Order.
-

Manager, Administrator
Please join us in welcoming our newest IRB Manager and Administrator.
-

IRB Guidance Updates
The IRB regularly reviews our guidance documents to make updates as necessary. Several updates have been made this year.
-

Associate Director
Please join us in welcoming our new IRB Associate Director.
-

ERICA Update: Edit Personnel Activity
The Edit Personnel activity has now been restored in ERICA. It has been modified to align with the new model for study personnel roles in the ERICA application.
-

Delay on Changes to the Common Rule
The Department of Health and Human Services (DHHS) has announced a delay on implementation of the proposed changes to the Common Rule.
-

ERICA Update: New Model for Study Personnel Roles in the ERICA Application
Effective January 13, 2018, the ERICA application changes the way it asks for study personnel to be listed. The new model is described on the IRB website, with an FAQ for new and existing studies.
-

IRB Staff Changes
Please join us in welcoming our new Panel 2 Coordinator.
-

IRB Director, Dr. Ann Johnson
We are pleased to announce that Ann Johnson will formally assume the IRB Director position as of October 15, 2017.
-

Recent Problem with IRB Expiration Notices
The ERICA system recently experienced a problem with the automated IRB Expiration Notices. We discovered that some studies were not receiving these automatically during the month of September, even though a study may have expired.
-

IRB Staff Changes
Please join us in welcoming our two newest IRB Coordinators.
-

IRB Staff Changes
Margaret McFarland and Annie Huff have accepted positions on the IRB Administrator team.
-

IRB Staff Changes
We have added two additional Operations Managers to the IRB.
-

Two-Factor Authentication Required for ERICA
Starting on June 1st University employees, students, and committee members are required to use two-factor authentication (2FA) to login to the ERICA system.
-

SIRB Training Now Available Online
The online version of the Single IRB (SIRB) training module is now available.
-

ERICA Updates
Several changes have been made in the ERICA system.
-

Revised Requirements for Human Subjects Training
The IRB’s training policy for Human Subject Research training and Good Clinical Practice (GCP) training has been revised
-

IRB Staff Changes
We are pleased to announce that Hannah Owen has accepted the position of IRB Administrator.
-

SIRB Training Requirements
Effective February 1, 2017, the University of Utah Human Research Protection Program requires investigators and staff performing research under a SIRB model to complete SIRB training before the research can be activated at the University of Utah.
-

GCP Training Requirements
Effective January 1, 2017, the University of Utah IRB requires clinical investigators and staff listed on an IRB application funded by the NIH to have both human subjects training and GCP training before an IRB application will be approved.
-

IRB Staff Telephone Availability Interruption
The IRB office is currently undergoing construction. Several of our staff members have moved to temporary accommodations away from their desks and usual telephones while the remodel is under way.
-

Delayed Pre-Review Timelines
Due to new staff onboarding and training currently happening at the IRB, as well as a greater than usual amount of applications submitted in recent weeks, we are running several days behind schedule on pre-reviews for all applications.
-

IRB Staff Changes
Please join us in welcoming our new Panel 4 Coordinator.
-

IRB Staff Changes
We are excited to welcome a new Administrator to the IRB.
-

IRB Staff Changes
We are happy to welcome a new IRB Coordinator. We are also pleased to announce that Jessica Loayza has joined the Administrator team.
-

Delayed Pre-Review Timelines
Due to new staff onboarding and training currently happening at the IRB, as well as a greater than usual amount of applications submitted in recent weeks, we are running several days behind schedule on pre-reviews for all applications.
-

IRB Staff Changes
We have added two new positions to the IRB.
-

IRB Staff Changes
We are pleased to welcome a new Coordinator to the IRB.
-

IRB Staff Changes
We are happy to welcome a new IRB Administrator to the New Study pre-review team.
-

IRB Staff Changes
Please join us in welcoming our new IRB Clerk.
-

IRB Updates
Due to F&A increase to 36.5%, the IRB fee schedule is changing for industry-sponsored new studies, continuing reviews, and amendments. The new IRB fee schedule will be effective on July 1, 2016.
-

RDRC-HUS Workflow for Amendments
Effective 5/1/2016, the RDRC-HUS will now review all amendments related to radiation use within the ERICA system.
-

IRB Staff Changes
Please join us in welcoming our newest IRB Administrator.
-

IRB Updates
All studies conducted at Shriners Hospitals for Children in Salt Lake City are now required to obtain approval from Shriners Hospital leadership before the IRB will issue final approval of the study.
-

Institutional Conflict of Interest Policy
Beginning January 15, 2016, the conflict of interest review process for all New Study applications will include a review for Institutional Conflicts of Interest (ICoI).
-

IRB Staff Changes
We are pleased to announce a new Coordinator for Panel 2.
-

ERICA Updates
Effective Sunday, October 26, 2015, the ERICA system has been updated.
-

Updated Guidance for Genetic Research
The IRB has updated the guidance on Genetic Research to add information on the topic of disclosure of incidental findings.
-

University of Utah HRPP Receives Reaccreditation from AAHRPP
We are pleased to announce that the University of Utah Human Research Protection Program (HRPP) was awarded full reaccreditation by AAHRPP.
-

IRB Updates
The IRB will no longer require that the FDA Form 1572 be provided with submissions for investigational drug studies and the IRB will no longer consider new risks reported in updated IBs as possible unanticipated problems according to the IRB’s reporting policy.
-

ERICA Updates
Effective Sunday, May 24, 2015, the ERICA system has been updated.
-

Interim Policy on Institutional Conflicts of Interest (ICOI) Rule
The University of Utah has implemented an interim rule for addressing institutional conflicts of interest, effective May 1, 2015.
-

Revised VA Consent and Authorization Templates
The VA Consent and Authorization Templates have been updated in accordance with the November 12, 2014 version of VHA Handbook 1200.05.
-

IRB Staff Changes
We are pleased to welcome a new Coordinator to the IRB.
-

Forms for Conducting Research at the VA
Researchers conducting procedures at the Salt Lake City VA need to be aware of the additional administrative forms that may be required in addition to the IRB application.
-

Use of External Research Nurses
The IRB has received several requests to allow the use of external research nurses who have contracted with a study sponsor to conduct research procedures with participants in their homes.
-

IRB Staff Changes
We are pleased to welcome a new Coordinator to the IRB.
-

IRB Staff Changes
Yan "Kiki" Wai, IRB Coordinator for Panel 2, has left the IRB. We are pleased to announce that Chantyl Staheli has been selected as the new IRB Coordinator for Panel 2.
-

Additional Exemption Categories
The IRB is introducing Exemption Categories 8-11 as additional non-federal categories.
-

VA Application and Administrative Review in ERICA
The VA administrative review has been incorporated into ERICA's new study review process.
-

Revised Consent and Parental Permission Templates
The IRB has updated a number of consent and parental permission document templates.
-

Changes to Tissue Banking Policy
Due to changes in HIPAA regulation released in March 2013, participation in the tissue bank must be optional for treatment/intervention trials.
-

Completing the Sponsor Section in ERICA
The IRB has reviewed studies where the sponsor section of the application is blank, even when the study has a sponsor.
-

Changes to Exempt Research Policy
This policy change proposes one additional non-federal exemption category, and outlines the new Exemption
-

IRB Updates
Effective Monday, April 29, 2013, the ERICA system will be updated.
-

New IRB Coordinators
The IRB is pleased to announce two new Coordinators have been selected!
-

IRB Staff Changes
There will be several IRB staff changes in the coming weeks.
-

Increasing Fees for Industry-Sponsored Studies
The IRB fee schedule for industry sponsored studies is changing.
-

IRB Updates
Effective Monday, June 18, 2012, the ERICA system was updated.
-

FDA Inspection of the IRB
The IRB was inspected by the FDA May 14 through May 18.
-

Protocol Summary Integration: FAQ
The IRB is continually monitoring the progress of the recent protocol summary integration update in ERICA as study teams complete new studies, amendments, and continuing reviews. We feel that the process is working smoothly as we address questions and concerns.
-

IRB Website Updates
The University of Utah Institutional Review Board is excited to announce that the IRB website has been updated.
-

ERICA System Offline
The ERICA system will be offline starting Friday, 1/20 at 12:00 PM until Monday, 1/23. Beginning 1/23/2012.
-

Protocol/Research Summary Forms
The IRB recently announced that the information collected in the Protocol/Research Summary forms is being integrated into the ERICA new study application.

